At AdviQual, we are more than consultants; we are your strategic partners in the healthcare industry's future. Founded in 2015, AdviQual has quickly risen to become a beacon of innovation and excellence in the medical device and in vitro diagnostic device sectors. Our mission is to empower manufacturers with the knowledge, strategies, and tools needed to navigate the complex landscape of healthcare regulations and market demands successfully.
• Quality Management Systems that ensure your operations meet and exceed international standards.
• Regulatory Compliance Consulting to navigate the complex regulations in global markets.
• Product Testing and Validation that proves the efficacy and safety of your products.
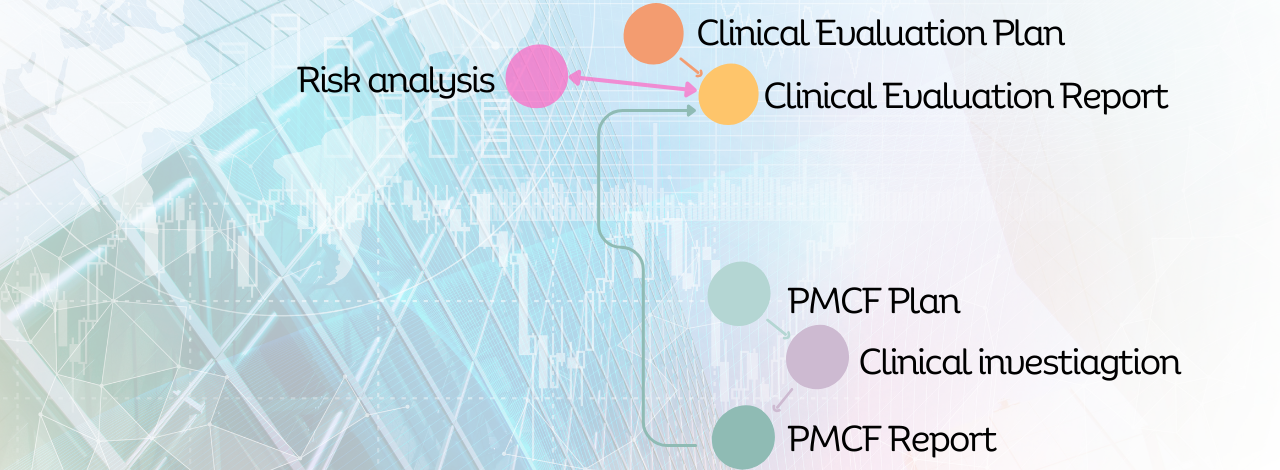
• Clinical Research Design and Management to generate compelling evidence of your product's value and effectiveness.
• Customized Solution: Strategies tailored to the unique needs and objectives of our clients.
• Global Compliance: Mastery of international standards and regulations.
• Result-Oriented Approach: Focused on maximizing your success in the market.